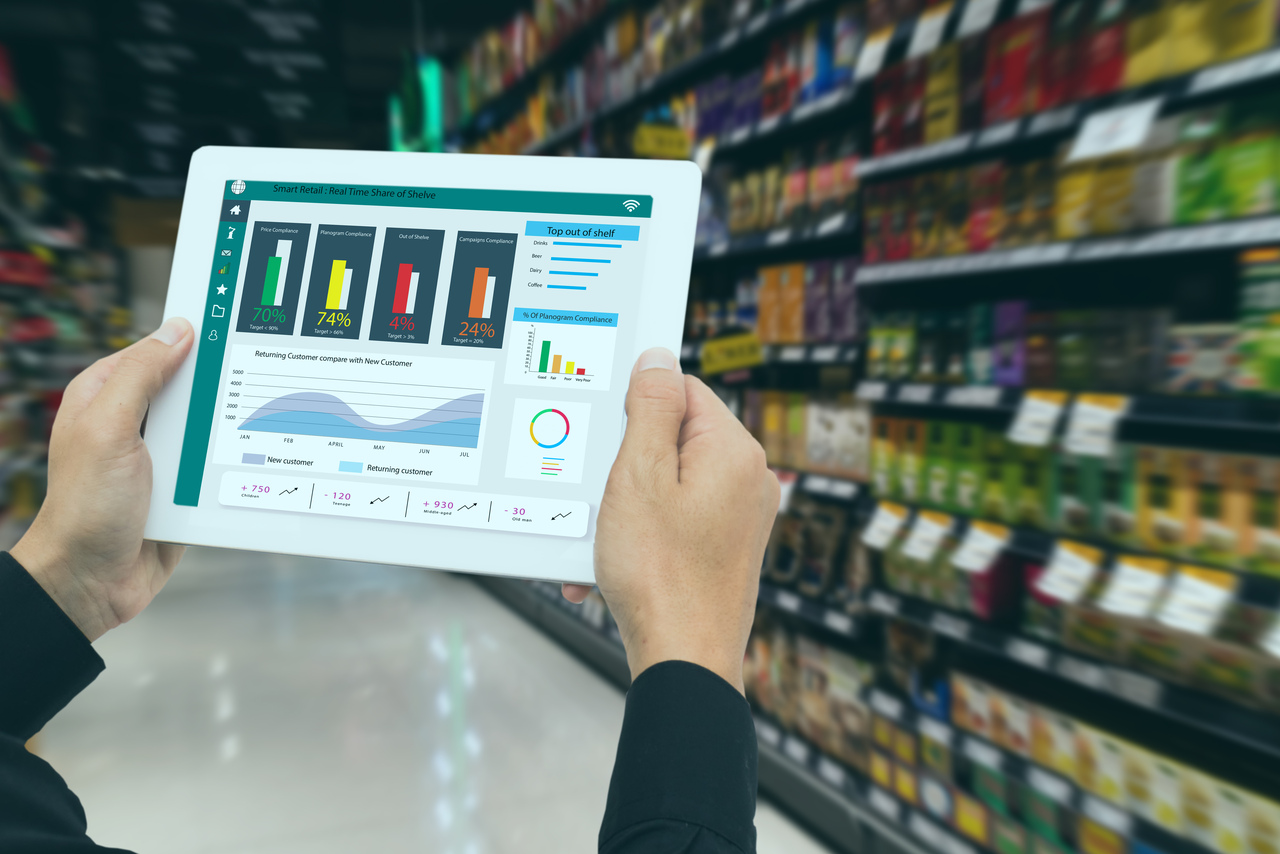
In the highly regulated Life Sciences industry, maintaining compliance is no longer just about meeting regulatory expectations, it’s about balancing patient centricity and operational excellence. As the industry accelerates toward digital transformation, AI integration, and connected systems, the need for a proactive and intelligent Audit Assurance Framework has become critical.
Focusing only on compliance and overlooking structured assurance mechanisms can lead to significant patient safety risks, compliance gaps, data integrity failures, delayed market access, or even product recalls. A modern, technology-enabled audit assurance model ensures not only inspection readiness but operational excellence, aiming at constant improvement as well as business sustainability.
Why Audit Assurance Matters in the GxP Landscape
Conventional audit approaches often fall short of providing assurance that a non-compliance would not recur. This is because the objective of closing an audit finding rather than understanding the absolute root cause, eliminating it through risk based methodology considering risk revolving around patient safety, drug quality and data integrity.
Hence, at Infosys, we believe in providing assurance that a non-compliance will be taken care of using appropriate controls with balancing regulatory ecosystem between patient centricity and operational excellence.
Our Methodology: Building Assurance with Purpose
1. Collaborate with Business and Quality Teams
We work hand-in-hand with Quality, Manufacturing, IT, and Digital teams to co-develop audit strategies. Our focus is identifying process and system risks early before they escalate into compliance gaps.
2. Process and Risk Mapping
Each GxP process (Manufacturing, Laboratory, Distribution, Clinical, CSV, CQV etc.) is mapped to potential risks. These are aligned with corresponding controls across Quality Management Systems (QMS), Data Integrity, and Digital Operations.
3. Assurance Lifecycle Advisory
From design to execution, we guide organizations through the entire audit lifecycle ensuring risk-based coverage, consistency across sites, and traceability of findings to CAPA and continuous improvement.
4. Audit and Control Design
We define and implement preventive and detective controls within GxP processes and digital systems (ERP, MES, LIMS, eQMS, etc.), balancing regulatory rigor with business practicality.
5.Inspection Readiness and Return-to-Service Audits
Our proactive inspection readiness program prepares organizations for regulatory and internal inspections with real-time insights and mock audits. Return-to-service audits ensure compliant requalification after major shutdowns, upgrades, or deviations.
6.Continuous Monitoring and AI-driven Insights
Leveraging digital dashboards, analytics, and AI-based risk models, we monitor compliance health in real-time predicting audit focus areas, identifying potential deviations, and strengthening the assurance ecosystem.
We also offer regulatory foresight dashboards that proactively track and predict upcoming changes in global regulations – such as the EU AI Act, FDA Digital Health Guidance, and ESG mandates—helping clients stay ahead of compliance requirements
7.Digital Maturity & Transformation Readiness
We assess client’s QMS, IT systems, and infrastructure to ensure they are future-ready for digital health, AI adoption, and Industry 4.0 transformation – minimizing disruption & maximizing scalability.
We benchmark client’s digital systems against global best practices.
Our Strategic Enabler: The Integrated Audit Assurance Framework
At the heart of our offering is the Integrated Audit Assurance Framework – a curated, continuously evolving library that connects risk areas to assurance checkpoints and controls across the GxP ecosystem.
It includes:
- GxP compliance controls (Manufacturing, QC, CSV, Data Integrity, IT Systems)
- Return-to-service and requalification audit criteria
- Risk score dashboard for compliance heatmap and trend analysis
- Self-assessment kit for maturity and capability evaluation
- Comprehensive audit checklist libraries with risk-weighted scoring
- AI and Digital system validation controls
- Inspection readiness trackers and regulatory audit playbooks
This framework acts as a living assurance system ensuring no critical compliance dimension is overlooked while driving consistency, traceability, and efficiency.
Conclusion: Embedding Resilience into the DNA of an organization to ensure continual assurance
Our Audit Assurance Service redefines this landscape through a structured, data-driven, and risk-based methodology aligning with global health authority expectations (USFDA, EMA, MHRA, TGA, WHO, etc.) and digital transformation initiatives.
By embedding audit assurance processes into the organization’s operational DNA, we enable proactive compliance, digital trust, and inspection readiness by design.
As pharma organizations evolve toward AI, automation, and cloud-driven ecosystems, audit assurance must evolve through resilience without compromise on assurance; especially with increasing regulatory scrutiny, faster digital adoption, and cross-functional dependencies, resilience is not an option — it’s a necessity.
Ready to transform from compliance to assurance landscape? Let’s start the conversation.