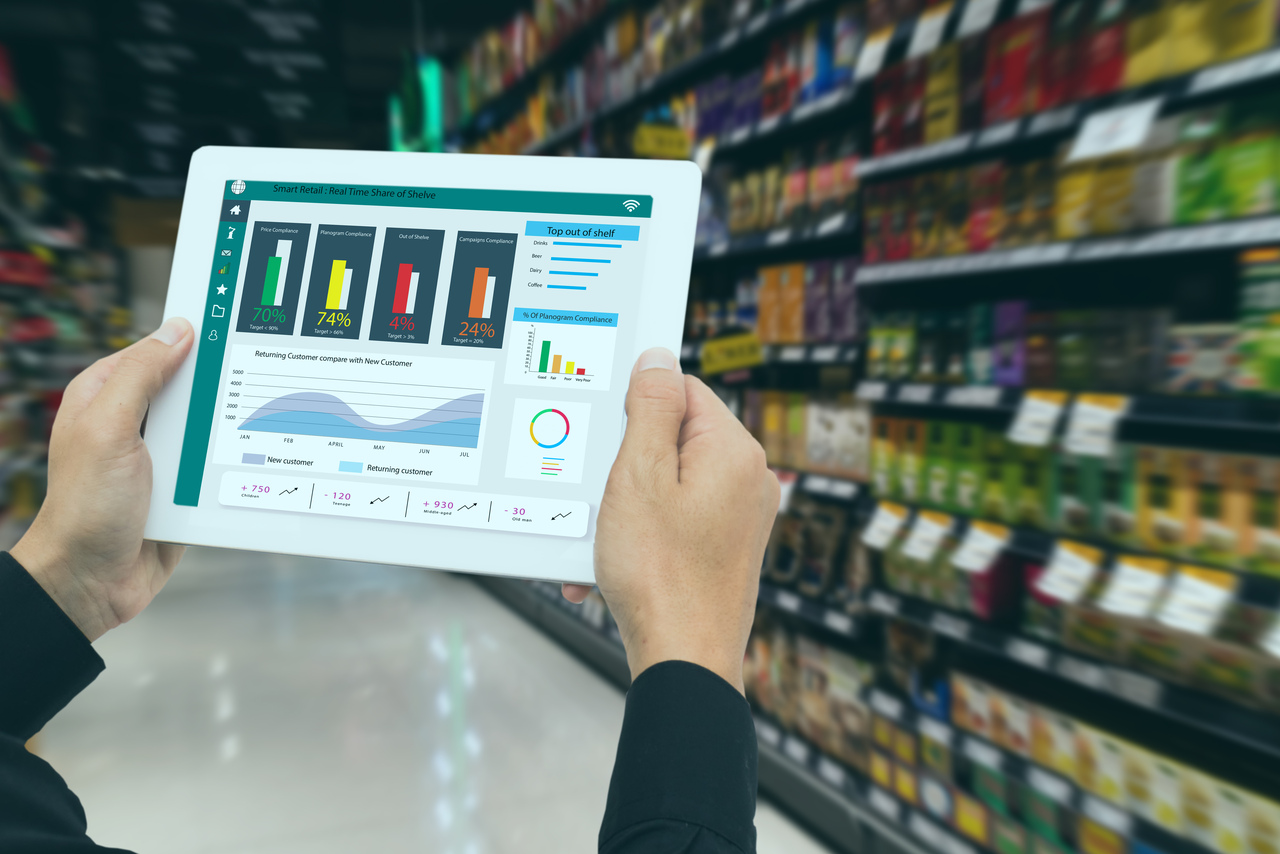
Healthcare is evolving rapidly, and so is the need for actionable insights that reflect real-world clinical practice. Traditional clinical trials offer controlled data, but they often fail to capture the complexity of patient journeys in everyday settings. This is where Real-World Evidence (RWE) steps in – reshaping how we understand treatment pathways, uncovering hidden trends, and enabling more personalized care strategies. By analyzing treatment patterns through RWE, stakeholders can validate guidelines, improve adherence, and design interventions that truly work in practice.
Why Treatment Pattern Analysis Matters – Navigating the challenges
Modern healthcare faces challenges that demand a data-first approach but faces hard-time in adoption of the same –
1. Off-label usage and therapy sequencing
In oncology and rare diseases, 20-30% of prescriptions are off-label, creating uncertainty in monitoring outcomes. RWE helps map real-world sequencing, ensuring compliance and better outcomes
2. Fragmented data sources hindering patient journey tracking
Over 60% of health systems struggle with interoperability, causing incomplete insights and ~15% revenue leakage from missed follow-ups; RWE integrates EHRs, claims, and registries into a 360° patient view, reducing care gaps and revenue loss.
3. Insufficient insights into regional and demographic treatment variations
Only ~50% of chronic disease patients (diabetes, TB, hypertension) adhere to therapy after 1 year, with adherence varying widely across regions; RWE highlights demographic and geographic treatment gaps, helping design targeted interventions to boost adherence.
4. Difficulty in assessing real-world effectiveness and safety
Post-marketing studies show up to 25% of drugs demonstrate different safety/efficacy in real-world vs. trials, risking costly withdrawals and regulatory penalties; RWE enables continuous monitoring, surfacing safety signals early and validating real-world outcomes.
Industry Trends Driving Adoption
- The healthcare industry is increasingly adopting RWE analytics for regulatory submissions, market access, and post-launch monitoring.
- AI/ML-powered predictive modeling: Platforms like Aetion Evidence applies advanced analytics to generate regulatory-grade RWE.
- Federated data networks: Solutions such as TriNetX enables global cohort comparisons without compromising patient privacy.
- Collaborative ecosystems: Partnerships among pharma, payers, and tech firms e.g., IQVIA Treatment Pathways and Flatiron Health are transforming how treatment pathways are visualized and optimized thereby improving real-world adoption strategies.
Recommendations
To fully leverage RWE-based treatment pattern analysis for better outcome
1. Invest in Data Integration:
Combine structured and unstructured sources for a unified view, revealing adoption trends and unmet needs.
2. Adopt Advanced Analytics:
Use AI/ML to uncover hidden patterns and predict outcomes, enabling smarter resource allocation and targeted trials.
3. Ensure Regulatory Alignment:
Design RWE studies that meet FDA/EMA standards, accelerating approvals and strengthening payer negotiations.
4. Promote Cross-Functional Collaboration:
Align clinical, commercial, and data teams early to translate insights into actionable strategies.
5. Focus on Patient-Centric Metrics:
Incorporate adherence, quality of life, and long-term outcomes to support value-based care models.
RWE-based treatment pattern analysis is no longer optional – it’s essential for improving patient outcomes and driving competitive advantage. By embracing integrated data strategies, advanced analytics, and collaborative models, healthcare stakeholders can move beyond assumptions and make decisions grounded in real-world practice. Organization should start building a data-first culture that turns insights into impact.