Blog Series Part 1
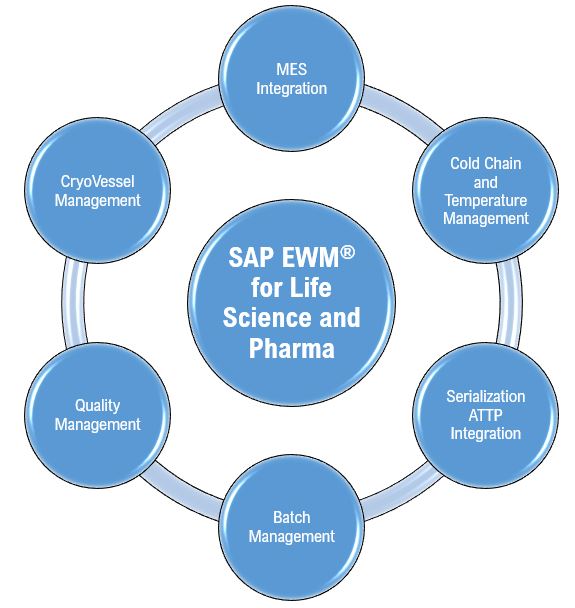
SAP EWM (Extended Warehouse Management) is a best-of-breed and best-in-class Warehouse Management System (WMS) product by SAP. This WMS product has the strong pedigree of its parent, it offers a great potential for life science and pharmaceutical companies to leverage upon inherently strong WMS capabilities to meet the process and functional requirements. I endeavor to share these insights using two blog series, in this first blog series, I have chosen the most challenging business areas of Production Execution, Serialization, and Cold Chain and Temperature Management.
A. MES-EWM Integration for Production Execution
The seamless integration of EWM with shop floor systems is a need of the day for an efficient end-to-end production execution. Shop floor Manufacturing Execution Systems (MES) requires a tighter integration to pull and push staging of components and raw materials to/from the warehouse. Goods Issue and Goods Receipt events in the MES for produced finished APIs, drug substances, and drug products require a signal to be sent to the warehouse system for the consumption and final storage, respectively. This functional process architecture is depicted in schematic# below.
SAP EWM offers out-of-the box APIs for connecting to external WM. Also, EWM has inherent capabilities like using standard collaboration messages, IDOCs, and BAPIs for integration of master data and transactional data with SAP Manufacturing Execution (SAP ME) and SAP MII (SAP Manufacturing Integration and Intelligence).
For non-SAP MES system integration, EWM offers out-of-the box API RFC enabled function modules*
(*Source, SAP Blog by Prakash Pol: https://blogs.sap.com/2020/12/22/sap-ewm-mes-direct-a2a-integration-via-api-for-receiving-hus-in-ewm/)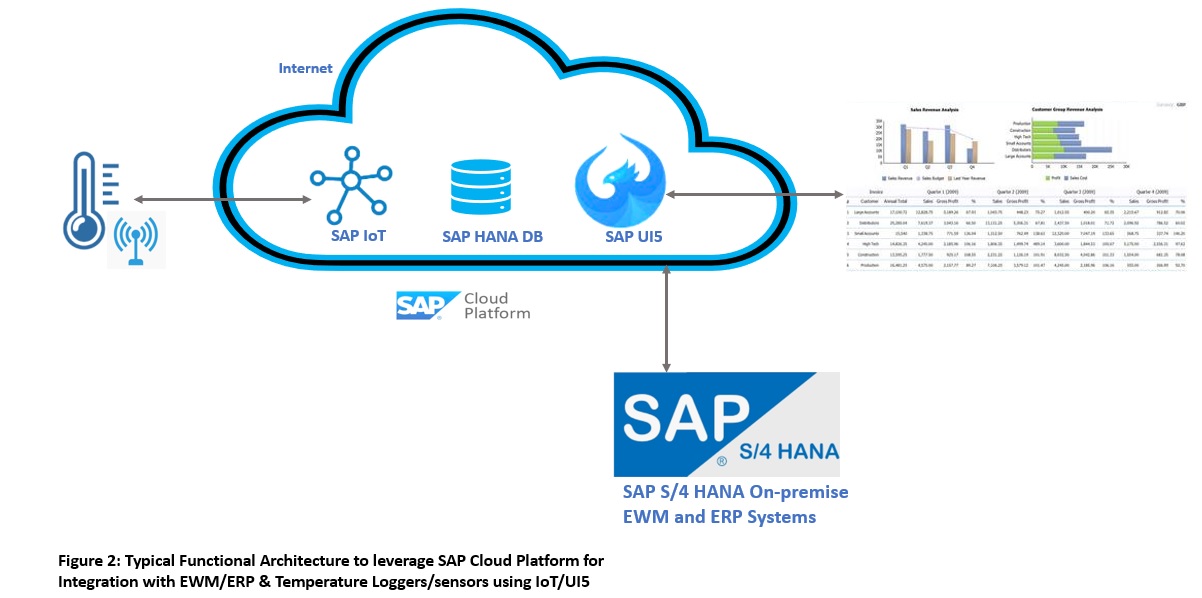
(#Source, SAP Blog by Prakash Pol: https://blogs.sap.com/2021/04/06/designing-a-robust-integration-between-sap-ewm-and-manufacturing-execution-system-mes/)
B. Cold Chain and Temperature Management with EWM
In the pharmaceutical and other life science industries, the manufacturing, warehousing, and supply chain processes like shipping require the active ingredients to be maintained at a certain cold temperature range for a defined period of time. This is also called cold chain management. Not only the cold Temperature Management (TM) is required but also thawing and acclimatization of APIs before used in production is important. Some of the key measuring parameters used here are TOR and TIR. Time-Out-of-Refrigeration (TOR), this clock starts ticking when the product is out of cold zones, and for Time-In-Refrigeration (TIR), this clock starts ticking when the product is moved to the cold zone. It is also required to record real-time events when temperature budgets and time tolerance is violated, also including events for example like when number thawing cycles are exceeded, the blocking of stock in shop floor or warehouse will be required with a quality inspection process. In certain business areas, for example in clinical trials, it is even required that serialized products are tracked and traced along with temperature monitoring.
Thanks to SAP’s new age of innovative products like SAP Cloud Platform (SCP) or SAP Business Technology Platform (BTP), it offers flexible and scalable architecture. These platforms offer components like SAP IoT (Internet of Things) for seamless connectivity over the internet with temperature data loggers having smart sensors. Technically, SAP EWM and MES systems can connect to SCP or BTP with API webservices and the core systems like SAP S/4HANA ERP production can communicate with OData services for transmitting the important product data for which temperature is monitored, for example updates of drug batches staged, produced, and released. To see the reporting of TOR/TIR/TM via the user interface, one can build a UI5 application to consume the data. This high-level conceptual functional architecture with its key components is depicted as below.
C. EWM-ATTP Integration for Serialization
Various global laws and regulations require pharmaceutical companies to adhere strictly to drug serialization and statutory reporting. SAP ATTP (Advanced Track and Trace for Pharmaceuticals) is such solution that offers serialization for drug and medicine products. Although there are various deployment models, including an add-on, the most recommended SAP deployment model for ATTP is to have one Centralized Repository System and integrate it with all the internal and external systems like EWM for warehouse processes impacting serialized products. the same is depicted in the below schematic.
Electronic Product Code Information Services (EPCIS) is a global GS1 Standard for creating and sharing track and trace drug and medicine serialization event data, both within and across global enterprises, to enable users an insight into physical objects like medicines, clinical and drug products. EWM can be technically integrated with ATTP, by exposing out-of-the-box provided FMs using OData (Open Data Protocol) API webservice, as a best practice. At the same time, it can give RFC (Remote Function Call) to fetch the serialized product data from ATTP for seamless integration. EWM sends various EPCIS events to ATTP whenever serialized products are undergoing warehouse transactions, some of the examples are mentioned below.
1. HU hierarchy changes due to consolidation and deconsolidation in serialized handling units should be reported to ATPP from EWM. These are reported by EWM by sending aggregation events with business steps packing and unpacking
2. Loading and unloading events like for example ATTP validating the EWM outbound delivery order and inbound delivery respectively linked to top-level Hus like Shipping Handling Unit (HU). These events are reported to ATTP by EWM upon status changes using object event with business steps of loading and unloading
3. Other ATTP events like for example Inbound returns to the warehouse

I will continue to share more insights in my second part of this blog series on the same topic – how SAP EWM product capabilities could help life sciences and pharmaceutical companies to leverage their key process areas in the warehouse management area.







nice and very useful information.