Blog Series Part 2
This is the second part of my blog series on how SAP EWM can be leveraged to support the industry-specific processes and needs of life science and pharmaceutical companies when it comes to warehouse management best practices and processes. SAP EWM is placed in the top leaders’ quadrant in the WMS product space (Source: Gartner June-2021). This is mainly on account of its product features that are suited as a best-in-class WMS solution and gaining popularity amongst SAP’s already wide customer base.
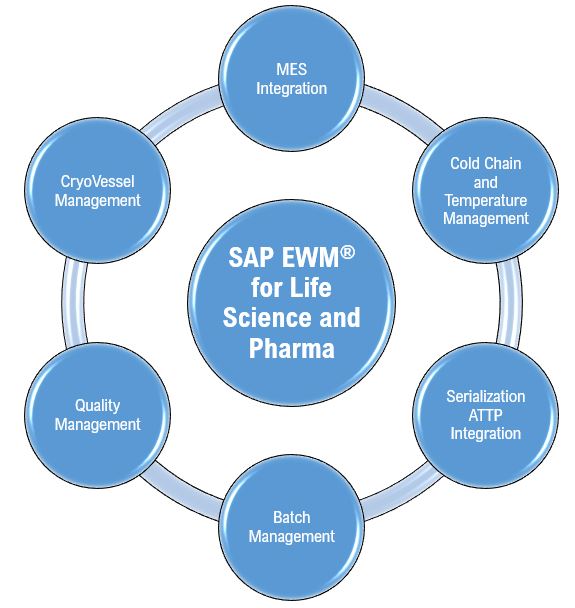
In my first blog, I have shared insights into various strengths of EWM into A. MES Integration, B. Cold Chain and Temperature Management and C. Serialization Integration using SAP ATTP.
In my continuous endeavor to unearth the great potential of EWM for the life science and pharmaceutical companies, in my second blog series, I have chosen another three challenging business areas of the industry in focus which are Batch Management, Quality Management, and Returnable CryoVessel Management.
D. Batch Management
In the life science and pharmaceutical industry, the common terminology used for batch management is Batch Disposition or Lot Disposition, this is rather a synonym with a Product Disposition.
If GMP (General Manufacturing Practices) and PQS (Pharmaceutical Quality System) are not well managed then the core batch disposition process will suffer and there will be a high chance of having bad drug products manufactured and circulated in the market, therefore there are strict regulatory and compliance frameworks involved and batch management is a vital part of the whole process to measure adherence to quality processes.
The typical pharmaceutical manufacturing process involves the depicted chevron sub-processes as below, the batch disposition is tightly integrated into the whole manufacturing process chain. Batch master data and batch records would generally have custom characteristics to represent batch disposition like the release, release with restriction, without limitation, rejected, blocked, not for human use, expired, returns, interim product, etc.

With EWM on SAP S/4HANA, the ERP batch master data is seamlessly integrated with S/4ERP. If it’s embedded EWM, the batch master is a commonly shared master object and need not be replicated and if it’s decentralized EWM on SAP S/4HANA, the batch master is distributed from S/4ERP to EWM via standard interfaces like using BATMAS IDocs and with the change pointers activated, batch master can be real-time synchronized.
It’s a very common scenario in the pharmaceutical business process, that depending on the Batch Disposition Status of APIs, raw materials, components the batches should not be determined or assigned to the warehouse tasks for staging, replenishment, or picking process, for example, batches which are in quarantine, released with some restrictions or blocked cannot be used. Such checks on batch disposition via status characteristic values are possible in EWM by implementing out-of-the-box Business Add-ins which are called during batch determination during warehouse task creation while stock removal/stock movements, the system can skip such batches which are not in desired batch disposition. If the overall status of the batch itself is restricted, EWM can prevent warehouse tasks for restricted batches from being created.
It is also common in pharma environment, to post goods receipt of Inbound materials to restricted batch, with EWM it is possible to do this by setting goods movement ‘Restricted’ indicator at the Inbound delivery document and item type level. If there is a shortfall in the remaining shelf life at the time of goods receipt, EWM can be configured to perform full prevention of GR and warehouse task creation or just perform a minimal check and do not reject or lock the item. It is worthwhile in a life science environment to implement an incompleteness profile by making fields for the remaining shelf life of batch mandatory or required to be entered.
E. Quality Management
EWM supports tighter integration of goods movement processes with the quality management component of ERP. It broadly has two main deployment options, i.e., Decentralized and Embedded. Decentralized-EWM uses the Quality Inspection Engine (QIE) to manage inspection documents, architecturally QIE is an additional software component in the SAP EWM system product. With Embedded-EWM in S/4HANA, the inspection lots are created and maintained by ERP QM to run quality management processes, and hence quality inspection documents are not maintained by EWM in parallel. Additionally, with Embedded-EWM, master data synchronization is not necessary as QM and EWM run in the same system. As of S/4HANA 1909 FPS1, Integration of Quality Inspection Engine to Multiple Enterprise Management Systems is also supported.

Using EWM, one can block the receipt of raw materials, components that are procured externally from a vendor, due to bad quality reasons. It is also possible to use acceptance sampling to inspect externally procured materials before the goods receipt can be posted in the Decentralized-EWM system. With Radio Frequency (RF) device, the warehouse operator can also perform a preliminary inspection of the handling units (HUs) in an EWM delivery to decide if the handling unit is of good quality or not.
Production pre-sampling for in-house manufactured drug substances and drug products can start even before the GR is posted from production to the warehouse stock in Decentralized-EWM on SAP S/4HANA, and hence the inspection result recording and usage decision can start even before inbound delivery is created in EWM.
There are some differences in terms of quality management processes supported by embedded versus decentralized-EWM. I have summarized some of the most relevant and useful quality management processes from a warehouse integration perspective in below table*
(*Source: RIN2021_EWM_Deployment_differences_V1 from SAP, Oct 2021)

In an industry environment like life science and pharmaceuticals, the batch managed product in the warehouse must undergo inspection at regular intervals even when it is stored in the warehouse bin, this is needed to check the physical status, condition, and efficacy of the batch disposition of an underlying product, this periodic inspection also strengthens the whole Pharmaceutical Quality Control system (PQS). EWM recurring inspection (IOT5) facilitate this requirement by enabling such periodic regular inspection of the material stored in warehouse bin and is treated as warehouse-triggered internal quality inspections which are not categorized as inbound or an outbound warehouse processes, but typically falls into warehouse internal process category. (Source: SAP Blog by Prakash Pol, https://blogs.sap.com/2020/05/26/warehouse-recurring-inspection-iot5-in-decentralized-ewm/)
F. CryoVessel Management
CryoVessel is a portable jacketed freeze-thaw vessel or tank which is used for transferring the produced Active Pharmaceutical Ingredient (API) that is further used as the main component to manufacture a drug product (which can be for example tablet, capsule, cream, injectable). These tanks must retain their ID throughout the end-to-end supply chain. The use of Handling Unit Management (HUM) in EWM is one of the most suited options to maintain the same unique HU or vessel ID number throughout its life cycle and the corresponding status of the vessel can be managed with the use of HU status like for example with status values of cleaned, filled, depleted. It would be best practice to use SSCC (Serialized Shipping Container Code) to represent Vessel ID so that GS1 standards can be followed across the end-to-end supply chain and logistics process of CryoVessel.
As depicted below, in a typical life cycle of a CryoVessel, the cleaning center will supply empty cleaned CryoVessel to API manufacturing sites with its Handling Unit number in EWM. In the next step, the frozen API is filled into the CryoVessel by the API manufacturing sites. The filled Batch of API can inherit the same Vessel HU number as that of the earlier received cleaned vessel while posting GR regarding inbound delivery where Source HU can be kept as destination HU. API site now ships this same unique HU to the drug product manufacturing site against outbound delivery. With the use of ASN transmitted from the API filling site, the Vessel HU number can be again kept the same while posting goods receipts at the drug product manufacturing site. After the consumption of API from the filled vessel by manufacturing the HU gets depleted status and it is then sent back to the tank cleaning center for the next cleaning process, this depleted tank transfer can again take place through the delivery mechanism to retain unique SSCC HU across the supply chain.
The CryoVessel are expensive containers, and they are stock managed, it needs to be tracked and traced across the supply chain. ATTP solution can be used for Vessel HU and drug product HU serialization tracking and with EWM-ATTP Integration the CryoVessel tracking is more seamless when the HU (Vessel) movements are posted in the EWM system during goods receipt and goods issue at cleaning centers, API site, and product manufacturing site. Moreover, the vessel equipment serial ID assigned can be maintained under API batch master data for individual Identification.

Conclusion:
SAP Extended Warehouse Management (EWM) can support a wide range of business applications in the life science and pharmaceutical industry. My First Blog and this blog in a series have selected the six most critical industry process areas, but there are certainly other equally important processes that could be very well supported using the inherent WMS capabilities of the innovative EWM product. SAP continues to expand its EWM solution roadmap and keeps adding new innovations in each release of SAP S/4HANA and it looks truly more promising than ever in every new EWM release.






Thank you Prakash! Much awaited blog. It was really helpful.
Nice blog about life science compliance software, I think your blog very helpful for more people. Thanks for sharing the information and check my new blog.