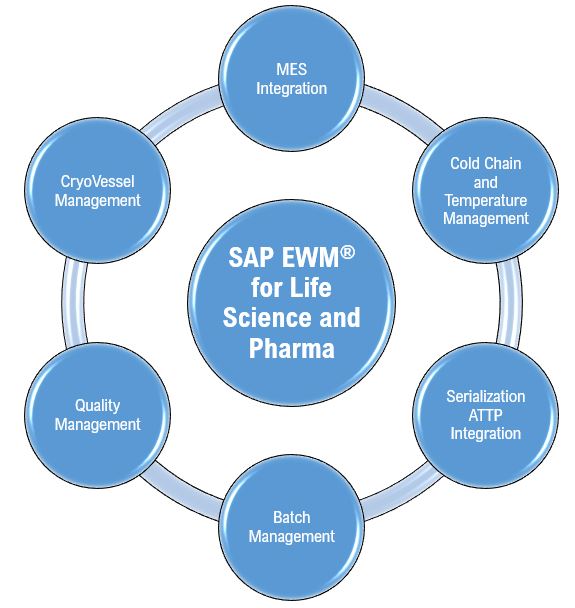
The life sciences industry is highly regulated and must follow stringent quality control. The batch release process is a critical part of the quality management function. It spans the release of the active pharmaceutical ingredients (APIs) for manufacturing through to the release of the drug product (DP) in the market.
There are several stages in the life cycle of a batch when quality processes play a critical role. Quality processes ensure the use of the right ingredients in the manufacturing of the Drug product while adhering to compliance norms.
Some of the key scenarios for quality management function are:
- Incoming inspection and release of APIs prior to manufacturing
- Incoming inspection and release of solvents, exipients etc.
- Release of primary pack (PP) and drug product after manufacturing
- Market release of the finished drug product to regional distribution hubs
- Distribution release of the finished drug product from regional hubs to local warehouses or customers
- Conditional batch release
While batch release process is similar across life sciences companies. However, lack of standardization, harmonization, and integration across business functions often leads to process inefficiencies and delays. Further, the data required for batch release decision residing on various applications makes the process time-consuming.
With dynamic market trends, evolving supply chains, and growing regulatory requirements, harmonizing, accelerating, and scaling the batch release process has become the need of the hour. On the other side, there are several challenges affecting the efficiencies in quality control processes.
Current Challenges:
In keeping with stringent quality and regulatory requirements, quality specialists perform several verifications on various parameters of each batch. While this helps them make informed decisions, many tasks are manual. Secondly, having a good overview of several data points across business functions is vital. However, information is scattered across several applications in the dispersed system landscape, increasing the potential for human error.

The dependency on multiple business functions and personnel for accurate information results in long lead times for information collection and decision-making. Further, quality teams may not have easy access to the data they require. The quality processes must be highly mechanized to detect, flag, and prevent human error. However, with systems gradually built over time, quality control may face challenges from a limited perspective of the big picture, restricted access to tasks, and constrained timelines. These could impair their ability to understand the impact of delays or errors on their end.
To address these challenges, life sciences companies must focus on few imperatives that can bridge the gap to set up a seamless and efficient batch release process.
Imperatives:
Pharmaceutical manufacturers need to set up standardized and harmonized quality processes across organizations and geographies.
Companies can achieve this by building a user-friendly, intelligent solution to bring all batch-related information on a single platform and help quality managers make informed decisions. Automation must be leveraged to improve the accuracy of batch usage decisions, minimize human errors, and enhance operational efficiency. This will also help de-risk, speed up, and scale up the entire batch release process. An end-to-end solution extended across organizational entities and geographies in an agile, seamless, and accelerated manner is the need of the hour.
While addressing process-related challenges, it is essential to ensure a best-in-class user experience through standardization. This can help accelerate solution adoption at scale.
Pharma manufacturers need state of the art solutions to address the challenges and to facilitate continuous improvement journey to meet the requirements of the future. Let us explore those in the next blog.