Stability Testing in the pharmaceutical industry refers to the inspection/monitoring of the quality of drug products as per the defined guidelines of the various regulatory bodies over a period of time. This ensures that the products retain its quality over the period. It also ascertains the shelf-life and storage conditions of the products. It is to be ensured that the product efficacy related to the quality and safety remains the same throughout the defined shelf-life. The acceptance and the subsequent approval of the product is governed by it’s efficacy.
Mainly it is designed for monitoring and evaluating the quality of API (Active Pharmaceutical Ingredients) and FP (Finished Products) over a period under the influence of various factors such as:
· Packaging Materials/Methodology
· Ambient Temperature
· Humidity / Moisture
· Light
· Container Orientation
The process of Stability Testing is initiated with the inception of a Stability Study which is driven by Protocol (can also be referred as Study Plan). The Stability Protocol has four key elements, viz. Orientation, Timepoint Conditions and Formulations.
Types of Stability Studies:
As per USP, the different types of Stability Studies at a high level that should be conducted are as follows:
· Physical stability – The physical stability may affect the uniformity and the release rate. Properties such as color, dissolution, palatability, appearance etc. should be retained.
· Chemical stability – Resistance to the decomposition in the drug (or it’s API) due to reactions occurring due to atmospheric and temperature changes.
· Microbiological stability – It’s the tendency to resist the microbial growth. Certain antimicrobial agents are used during the preparation of the drug in defined limits. These agents maintain the effectiveness of the drug for the period.
Why Stability Studies:
· Stability Study is largely used to provide data to support clinical trials and for the registration submission.
· Developers can make any adjustments to the formulation if required.
· Determine the requisite condition requirement for the storage of the product.
· To ensure that any probable issues are identified upfront during the development of the formulation.
· Determine and establish the expiry date for the product.
Life Cycle of Stability Study in Development of a New Drug Product:
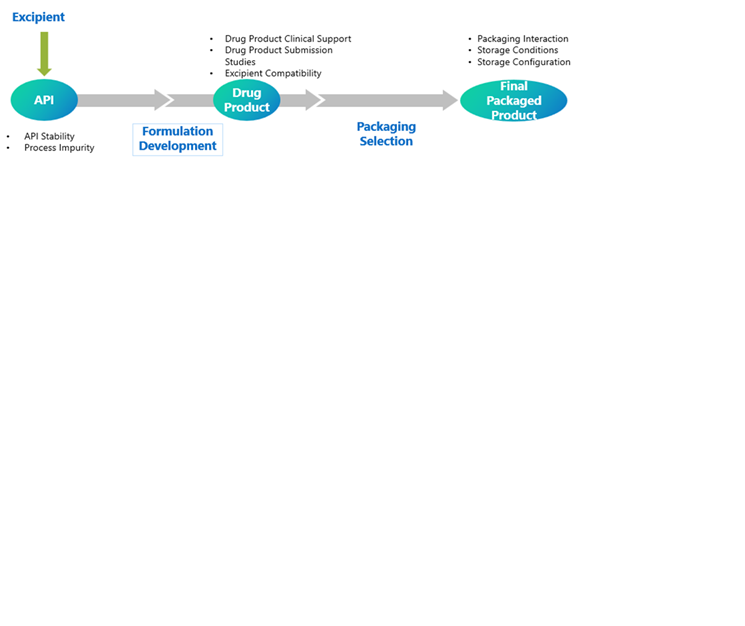 Different Stages of Stability Testing:
Different Stages of Stability Testing:
From a broader perspective, the different types of Stability Studies Trial Design are as below:
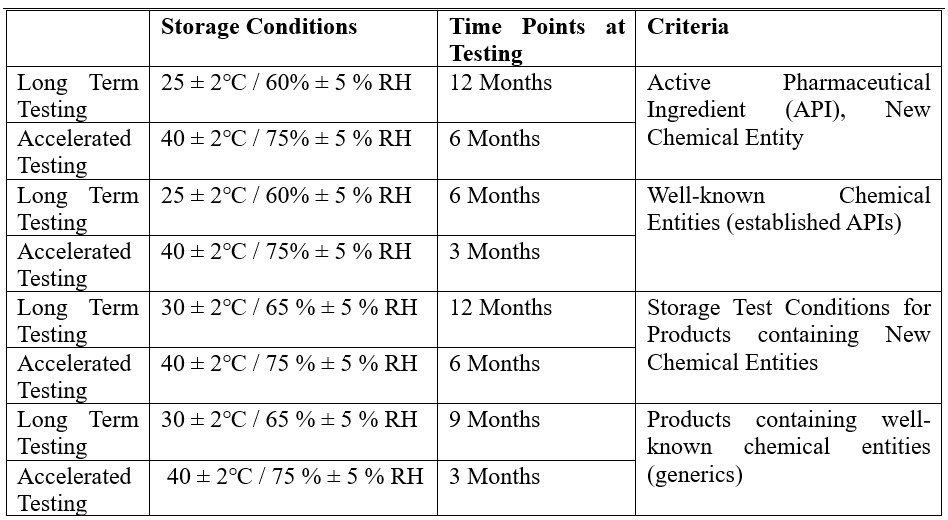
Typical Stability Workflow:
A typical Stability Workflow consists of the following steps:
· Creation of Stability Study – The request is usually initiated by the Lab QA for respective Lots (for Finished Products, Raw and Packaging Materials in Manufacturing Process) and for respective new Products (during Formulation Development).
· Designing and Approval of Protocol – The Protocol is the cornerstone of the Stability process and usually defines the combination of Orientation, Conditions and Timepoint for Stability Testing. It’s also a critical part of the Inventory required for the Study. Once a Protocol is approved, any modifications to it requires an Amendment to the study.
· Charging and Initiating the Study – Charging of a Stability Study requires pulling the requisite amount of Inventory for the entire Study and storing it in various Storage Chambers as per the Conditions defined in the Protocol.
· Testing of Samples – Once the respective timepoints defined in the Protocol are reached, certain amounts of Samples are pulled from the Storage Chambers for testing of the Samples.
· Data Review – After the completion of the Testing and capture of the analysis results, the data is sent for review where it can be Approved, Rejected or sent for any additional or retesting, if required.
· Submission – The Stability Summary Report is generated after the Approval of the analysis result and sent to the concerned authorities as required.

AI in Stability:
AI is used to analyze the historical data of the product to predict the potential stability issues, identify trends, and optimize formulations. This enables faster and accurate assessment of the product’s shelf life often by leveraging ML algorithms to identify patterns which might go undetected to human analysis. Enablement of AI leads to quicker & improved development cycles, cost reductions, and reduced TAT to market.
Common Applicability of AI in Stability:
· Predictive modelling analyzes the historical data of various formulations and their attributes. AI can assist in predicting how a new drug might perform under various conditions.
· Accelerated Testing helps analyze data for extreme conditions (high temperature, humidity) to extrapolate results and predict the long term stability. This reduces the time required for testing by extrapolating the results.
· Data analysis on various ingredient combinations leads to Formulation Optimization. The optimal formulation design helps in maximizing the shelf life and assists in minimizing the product degradation.
· Sending alerts on Real Time Monitoring of Storage Chambers for parameters like temperature and humidity, when integrated assists in managing any deviations and enabling corrective actions.
Conclusion:
In the dynamic landscape of pharmaceutical stability testing, it ensures the well-being of patients by establishing the accuracy of the shelf-life and storage conditions of the medicinal properties. It is one of the guiding tool for the drug developers success. Thorough understanding of the Stability Testing process is a must to understand the details of the physical, chemical and microbiological aspects of any formulation. Conceptual understanding of the different Stability types helps in the initiation of the innovations to transform a patient’s life and maintains the standard towards drug excellence.