Regulatory Enablement Through IDMP: Strengthening Global Drug Safety and Efficiency
The Identification of Medicinal Products (IDMP) is a comprehensive framework of five ISO standards designed to uniquely define and describe medicinal products across the globe. These standards encompass key elements such as product and pharmaceutical identification, substances, dosage forms, routes of administration, and units of measurement.
At its heart, IDMP aims to:
- Standardize terminology and documentation, and
- Enable seamless data exchange among regulators, manufacturers, suppliers, and distributors worldwide.
This harmonization is vital for pharmacovigilance, ensuring that medicinal products are accurately tracked and monitored throughout their lifecycle to safeguard patient health.
Why Centralization Matters
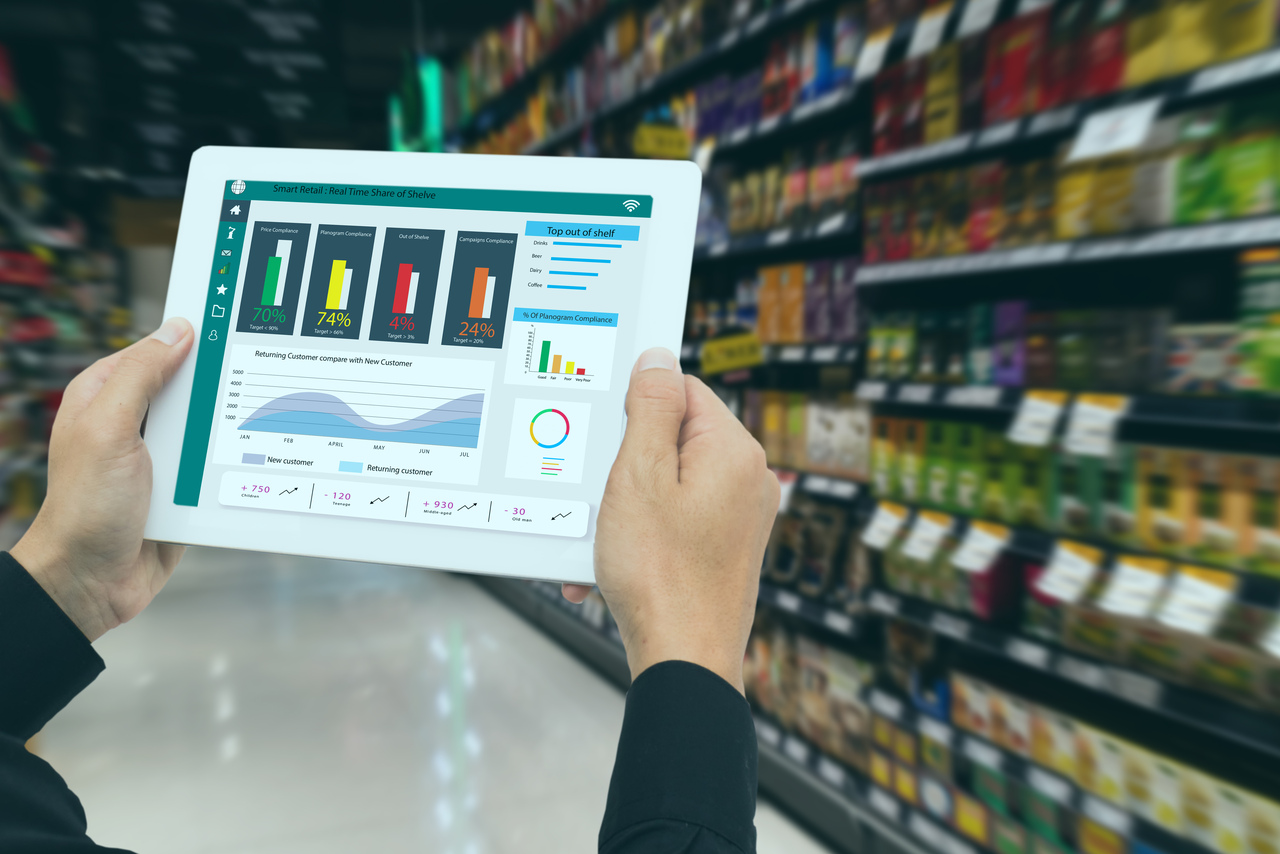
Centralizing IDMP-related processes can significantly boost both regulatory compliance and operational efficiency. By consolidating data management efforts, pharmaceutical companies can create a single source of truth for product information. This reduces inconsistencies, enhances data quality, and improves cross-functional collaboration across R&D, clinical trials, regulatory affairs, and supply chain operations.
Moreover, centralized systems simplify the submission and maintenance of product data, making it easier to meet evolving regulatory requirements and respond swiftly to safety concerns.
The Strategic Value of IDMP Implementation
Adopting IDMP standards—especially when paired with centralized interventions—offers a powerful solution for managing medicinal product information. It not only strengthens patient safety through accurate and consistent data but also drives internal efficiencies and fosters better communication among stakeholders.
Ultimately, this approach supports a more transparent, agile, and reliable global healthcare ecosystem, positioning organizations to meet regulatory demands while delivering greater value to patients and partners alike.