EUDAMED represents a seismic shift for the medical device industry, transforming regulatory compliance from a fragmented, document-centric task into a unified, data-driven mandate.
For most companies, achieving full EUDAMED integration isn’t just about submitting data; it’s about fundamentally overhauling decades of legacy processes and systems.
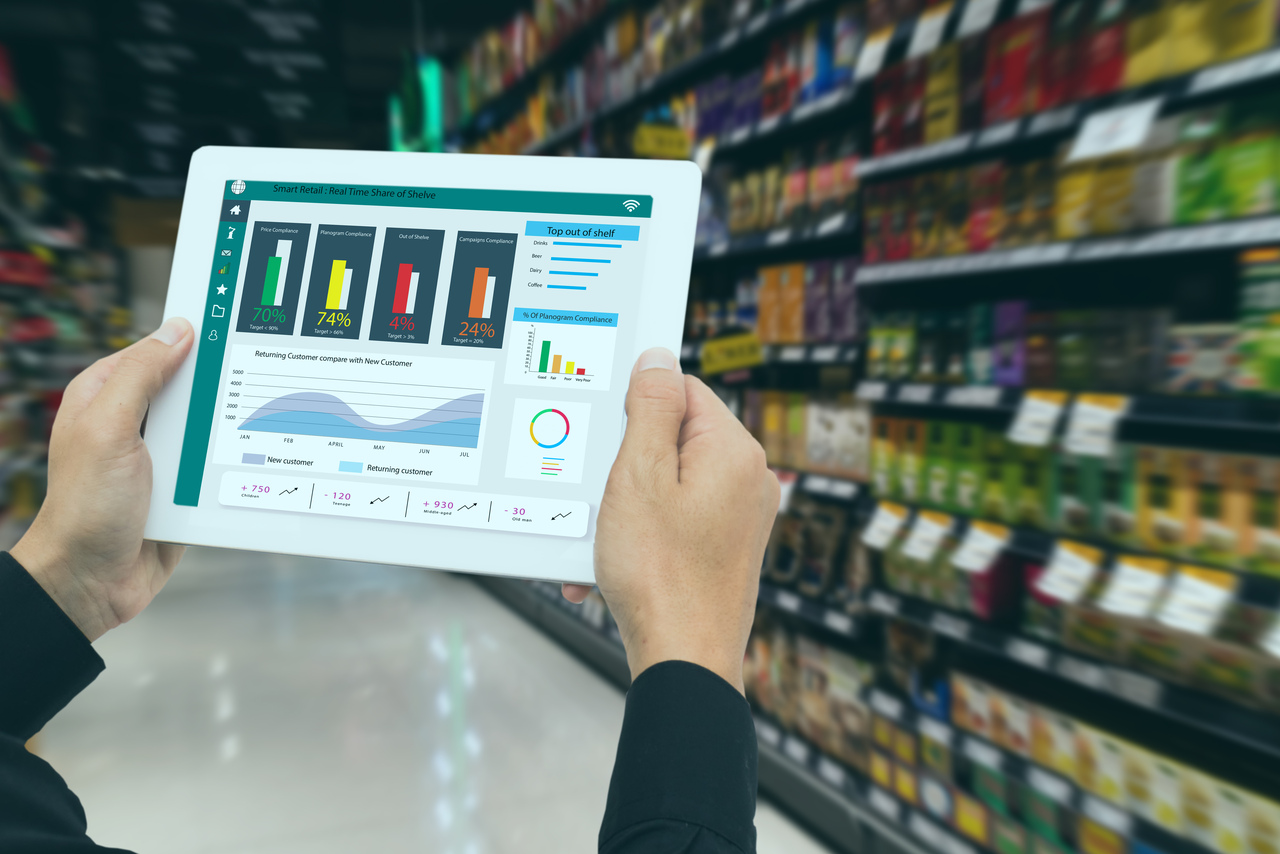
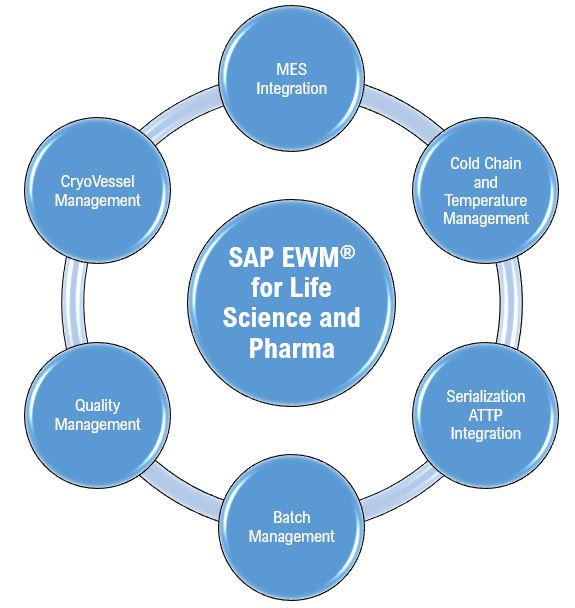
The real challenge lies in this transformation, moving from disparate data silos and manual workflows to a centralized, automated, and governed data ecosystem. A reactive, “compliance-only” approach will lead to immense administrative burden, but a proactive, “data-first” strategy will turn EUDAMED into a powerful enabler of operational efficiency, enhance patient safety, and strategic advantage.
Read more in our latest point of view here: EUDAMED Imperative- Transforming Legacy to Modern with Data First-Approach